Brain Protein Discovery Opens New Path for Memory Loss Treatment
Lowering It Boosts Cell Regeneration and Cognitive Function
Restoring memory and mental sharpness in older adults requires more than just preventing disease — it also means decoding the subtle molecular changes that occur in the aging brain. This insight comes from a report published by New Atlas, citing groundbreaking research featured in Nature Aging.
Cognitive Decline: More Than Just Aging
Contrary to the common belief that memory loss is an inevitable part of getting older, scientists now know that age-related cognitive decline isn’t merely the result of the natural passage of time. It often happens because neurons either die off or lose their ability to fire effectively at synapses — the tiny junctions where signals pass from one nerve cell to another.
For years, researchers have investigated many molecular changes that occur as we age. Only a few, however, have been identified as direct drivers of cognitive decline. Among the leading suspects has been the accumulation of iron in the brain, which is known to damage neurons and interfere with communication between brain cells.
Iron Accumulation and Slower Thinking
One study tracked the buildup of iron deposits in the brains of elderly individuals and found a strong correlation with slower thinking and memory problems. Another study provided even stronger evidence linking iron accumulation with Alzheimer’s disease, showing that high iron concentrations could accelerate the formation of damaging plaques and tangles in brain tissue.
Driven by these findings, a team of researchers at the University of California set out to pinpoint the exact molecular culprits that trigger age-related memory decline — and, more importantly, to find a way to stop or even reverse it.
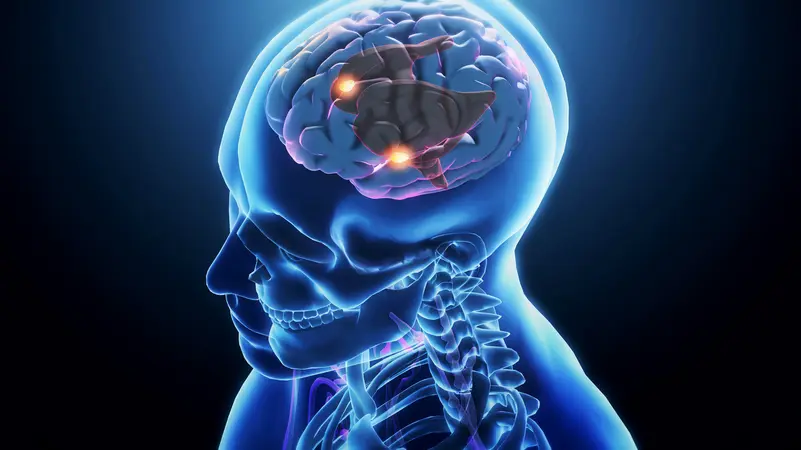
Spotlight on the Hippocampus
The scientists focused on the hippocampus, a small yet critical brain structure responsible for learning and memory. The hippocampus is also one of the most vulnerable regions when it comes to aging. Using a sophisticated technique called single-nucleus RNA sequencing, the team was able to examine gene activity in individual neurons.
What they found was striking: a protein known as Ferritin Light Chain 1 (FTL1) — a molecule closely tied to iron storage — appeared in much higher quantities in the hippocampus of older mice compared to younger ones. This excess of FTL1 was associated with weaker neural connections and reduced cognitive performance.
Turning Young Brains Old — and Back Again
To confirm their findings, the researchers conducted a fascinating experiment. They artificially increased FTL1 levels in the brains of young mice. The results were dramatic: the animals’ brains began behaving like those of much older mice. Their neurons formed fewer connections, their memory performance dropped, and their neural networks became noticeably less complex.
In lab dishes, neurons exposed to excess FTL1 grew abnormally short and had fewer branches, forming stunted networks instead of the rich, interconnected webs seen in healthy young neurons.
But the real breakthrough came when the scientists tried the opposite approach. By reducing FTL1 levels in older mice, they were able to rejuvenate their brains. The neurons began reconnecting, memory function improved, and the hippocampus appeared to regain youthful energy. In other words, the brains of these older mice seemed to “remember” how to be young again.
A Clever Metabolic Solution
Lead researcher Saul Villeda explained that FTL1 doesn’t just impair memory — it also slows down metabolism inside hippocampal cells. The team discovered a clever workaround: when they treated these cells with a compound that reactivated their metabolic processes, the damage was prevented.
This discovery opens the door to a completely new class of therapies aimed at restoring brain function in aging populations. By targeting FTL1, scientists hope to spark regeneration in brain cells and improve memory and learning abilities in older adults — potentially offering new hope for those at risk of dementia and Alzheimer’s disease.
A Glimpse Into the Future
The findings suggest that age-related memory decline may not be as irreversible as once thought. If similar results can be replicated in humans, it could revolutionize how we approach cognitive health and aging. Instead of focusing solely on symptom management, future treatments could directly reverse the biological changes that cause memory loss in the first place.
As researchers continue to refine their understanding of FTL1 and its role in brain aging, the possibility of brain “rejuvenation” may move from science fiction to medical reality.